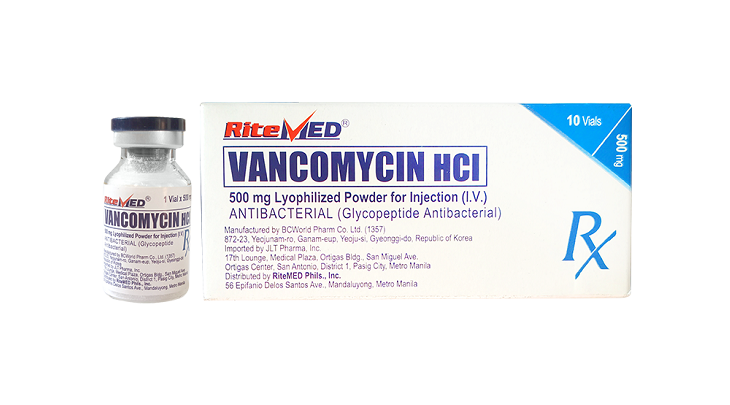
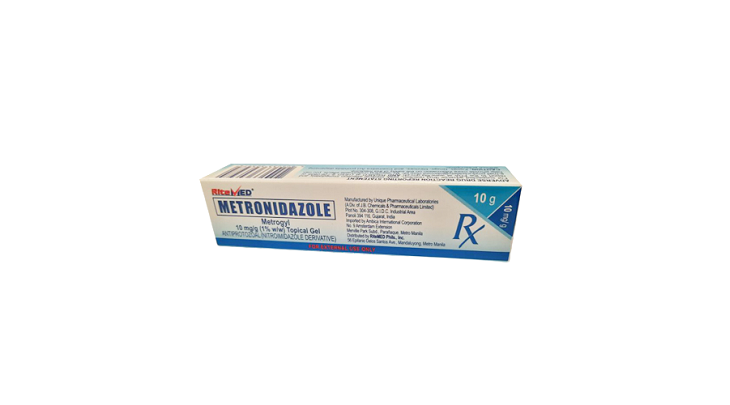
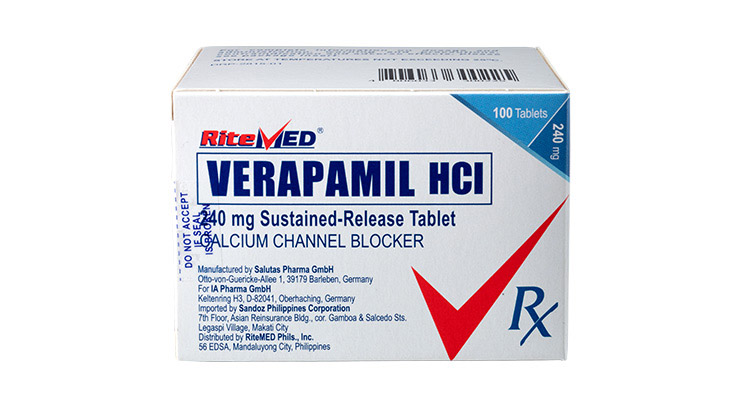
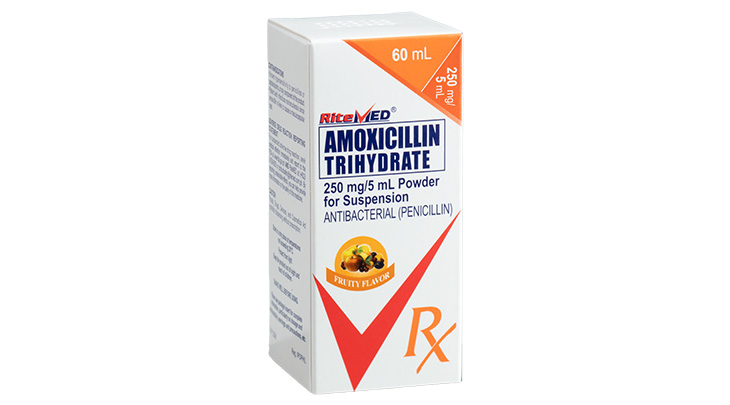
Product Details
What is this medicine used for?
Cetirizine hydrochloride is used as a symptomatic relief of allergic conditions including rhinitis and chronic urticaria.
How much and how often should you use this medicine?
6 mos. to 2 years old: Initial dose 2.5 mg (1/2 teaspoon) per day. This may be increased to a maximum of 5 mg (1 teaspoon) per day, given two times per day.
2 to 5 years old: 2.5 mg (1/2 teaspoon) twice a day or 5mg (1 teaspoon) per day as one dose
Or, as prescribed by the physician.
Warnings and Precautions:
Cetirizine hydrochloride should be given in cautions in patients who require mental awareness of physical coordination in their work such driver or machince operators since it may cause drowsiness. Use in pregnant women no data is available on the safety of administration of Cetirizine hydrochloride in pregnant women apart from an animal study which reveals absence of teratogenicity. As such, Cetirizine hydrochloride must be given to pregnant patiens only when necessary. Use in lactation mother Ceterizine is excreted in breast milk. Thus, use in lactating mothers is not recommended. Use in infants infants and children may not be given Cetirizine hydrochloride as this may increase risk of experiencing antimuscrarinic effects. Use in Elderly patients Cetirizine hydrochloride must be given with caution to elderly patients who most likely have impaired renal and hepatic function. Since the drug i metabolized through the renal mechanism, half life of Cetirizine hydrochloride in such patients will be prolonged. Dose of drug must be reduced in elderly patients.
Undesirable Effects:
Cetirizine hydrochloride, though considered as a 'non-sedating' antihistamine, was found to cause drowsiness. Recurrent acute hepatitis developed in one patient taking Cetirizine hydrochloride for control of seasonal allergic rhinitis. Other adverse effects associated with the use Cetirizine hydrochloride are irritability, insomia, fatigue, dry mouth, pharyngitis dizziness, headache, abdominal pain, diarrhea, epistaxis, bronchospasm, nausea and vomiting and hypersensitivity manisfested by uticaria and fixed drug eruptions. Although Cetirizine hydrochloride has low potential for severe hepatoxicity, the possibility of developing autoimmune-mediated hepatoxicity should be considered when administering the drug. A patient under long-term treatment and Cetirizine hydrochloride for atopic dermatitis was reported to have developed life-threatining hepatitis.
Interaction w/ other medicaments:
Sedation may be enhanced upon concomitant use of anthisamines is general with the central nervous system depresssants such as barbiturates, alchohol, hypnotics, opioid analgesics, anxiolytic sedatives and neuroleptics. The use of Cetirizine hydrochloride with drugs that inhibit the cytochrome P450 microsomal enzymes such as azithromycin, erythromycin and ketoconazole, didi not cause clinically significant changes. Effects of alcohol and other CNS depressants are enchanced by Cetirizine hydrochloride. Pharmacokinetics priorities of ritonavir, HIV-protease inihibitor, are not affected by Cetirizine hydrochloride.