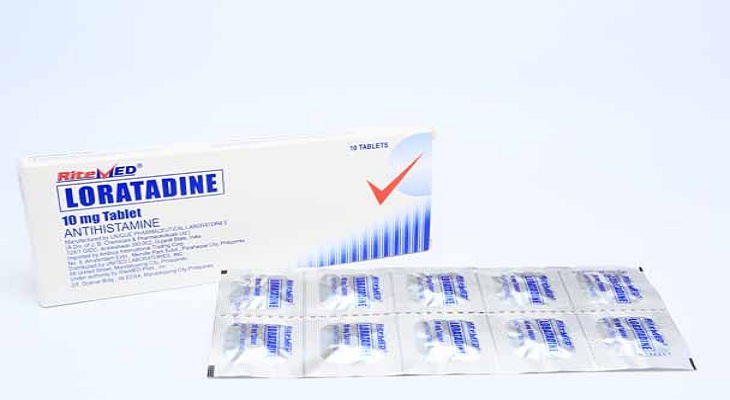
Product Details
What is this medicine used for?
Loratadine is indicated for the symptomatic relief of hypersensitivity reactions including allergic rhinitis, chronic urticaria and other allergic dermatologic disorder.
How much and how often should you use this medicine?
For adults, oral loratadine is given 10mg per day. For children, loratadine is administered as a single dose at 5 mg per day in children 2 to 12 years of age, <30 kg and 10 mg per day for those children 2 to 12 years of age, >30 Kg. Patients with hepatic failure or mild renal insufficiency are given loratadine 10 mg evey other day for adults and children 6 of age and order. Likewise, children 2 to 5 years of age who are having renal problems are given 5 mg every other day. Or as prescribed by the physician.
Warnings and Precautions:
Use in Elderly Patients in case of elderly patients who may have decreased liver and renal functions, peak plasma concentrations for the drug is increased and clearance of loratadine is altered. Thus, lower dose of the drug should be given to them. Use in children Once a day dose of loratadine has been proven effective and safe in children 3 to 12 years of age for the treatment of allgergic rhinitis. The safety of loratadine in patients younger than 2 years old is not yet established. Use in Pregnant Women USE FDA PREGNANCY FACTOR B (Animal reproductive studies have failed to demonstrate a risk to the fetus and there are no adequate well-controlled studies in pregnant women OR animal studies which have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the in any trimester.) Use of Loratadine in pregnant women is not associated with a large risk for major malformations. Administration of Loratadine during justifies the risk to the fetus. Use in Lactating Mothers Although the american Academy of Pediatrics has seen no adverse effects in infants on lactation, Loratadine must be administered with caution to patients who are breastfeeding mothers since the drug and its metabolites are excreted in breast milk. Use in Patients with Hepatic Impairment Patients with hepatic impairment are advised to consult their physicians before starting therapy with loratadine.
Undesirable Effects:
Adverse effects experienced with loratadine are usually mild and transient. Patients who are given loratadine at doses higher than 10 mg usually experience drowsiness, insomia, somnolence and dry mouth. The incidence of drowsiness is dose-related. Other side effects reported with the use of loratadine includes headache, fatigue, sedation in some nervousness, dysphonia, hyperkinesia, malaise, epistaxis, pharyngitis, altered salivation, altered taste, sinusitis, abdominal pain and other gastrointestinal side effects. Causes of severe necroinflammatory liver injury and abnormal hepatic function have been associated with the use of loratadine.
Interaction w/ other medicaments:
Concomitant use of loratadine with drugs that inhibits or are metablized by cytochrome P450 isoenzymes CYP3A4 and CYP2D6 such as ketoconazole, fluconazole, cimetidine, clarithromycin, erythromycin, quinidine, and flouxetine, increase plasma concetration in the blood. Although loratadine has shown significant pharmacokinetic interaction with co-administered with ketoconazole and cimitedine, it had no effect on electrocardiograph parameters (QTc). Likewise, elevated steady-state concentrations of loratadine and disloratadine with clarithromycin and erythomycin did not result to any adverse cardiovascular effects related to prolongation of the QTc interval.